|
 |
Tradename |
|
|
Trade names are indicative and excipients composition can be different depending on the country and manufacturers
|
|
Narop |
Sweden |
|
Naropéine |
France |
|
Naropin |
Argentina, Austria, Belgium, Brazil, Canada, Chile, Denmark, Egypt, Finland, Germany, Great Britain, Hungary, Iceland, Ireland, Italy, Luxembourg, Malaysia, Mexico, New Zealand, Poland, Saudi Arabia, Slovenia, Spain, Switzerland, United Arab Emirates, United States of America |
|
Naropina |
Italy |
|
Ropiconest |
Mexico |
|
Ropivacain |
Argentina, Belgium, Brazil, Canada, Chile, Croatia, Germany, Norway, Romania |
|
Ropivacaina |
Argentina, Brazil, Chile, Italy, Peru, Spain |
|
Ropivacaine |
Belgium, Canada, Croatia, Finland, France, Great Britain, Hungary, Ireland, Luxembourg, Norway, Sweden, Tunisia, United States of America |
|
|
 |
 |
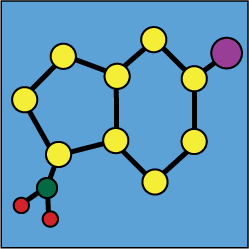
Stability of mixtures : Ropivacain hydrochloride |
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
1,5 mg/ml |
|
2-8°C |
| 51 |
 |
|
 |
1832

|
 |
 |
1,5 mg/ml |
|
20°C |
| 51 |
 |
|
 |
1832

|
 |
 |
1,5 mg/ml |
|
2-8°C |
| 11 |
 |
|
 |
1832

|
 |
 |
1,5 mg/ml |
|
20°C |
| 11 |
 |
|
 |
1832

|
 |
 |
1,2 mg/ml |
|
4°C |
| 180 |
 |
|
 |
4522

|
 |
 |
1 & 2 mg/ml |
|
30°C |
| 30 |
 |
|
 |
1665
|
 |
 |
1 & 2 mg/ml |
|
30°C |
| 30 |
 |
|
 |
1665
|
 |
 |
1 & 2 mg/ml |
|
30°C |
| 30 |
 |
|
 |
1665
|
 |
 |
1 & 2 mg/ml |
|
30°C |
| 30 |
 |
|
 |
1665
|
 |
 |
2 mg/ml |
|
30°C |
| 30 |
 |
|
 |
1665
|
 |
 |
2 mg/ml |
|
30°C |
| 30 |
 |
|
 |
1665
|
 |
 |
2 mg/ml |
|
30°C |
| 30 |
 |
|
 |
1665
|
 |
 |
2 mg/ml |
|
30°C |
| 30 |
 |
|
 |
1665
|
 |
 |
1 mg/ml |
|
21°C |
| 16 |
 |
|
 |
2051

|
 |
 |
1,2 mg/ml |
|
23°C-25°C |
| 30 |
 |
|
 |
1620
|
 |
 |
1 mg/ml |
|
40°C |
| 6 |
 |
|
 |
2051

|
 |
 |
1,2 mg/ml |
|
4°C |
| 30 |
 |
|
 |
1620
|
 |
 |
1 mg/ml |
|
4°C |
| 30 |
 |
|
 |
2051

|
 |
 |
6 mg/ml |
|
18-24°C |
| 14 |
 |
|
 |
3998

|
 |
 |
1 & 2 mg/ml |
|
25°C |
| 15 |
 |
|
 |
3780

|
 |
 |
2 mg/ml |
|
25°C |
| 15 |
 |
|
 |
3296

|
 |
 |
2 mg/ml |
|
4°C |
| 15 |
 |
|
 |
3296

|
 |
 |
1 & 2 mg/ml |
|
4°C |
| 15 |
 |
|
 |
3780

|
 |
 |
1,5 mg/ml |
|
2-8°C |
| 51 |
 |
|
 |
1832

|
 |
 |
1,5 mg/ml |
|
20°C |
| 51 |
 |
|
 |
1832

|
 |
 |
7.5 mg/ml |
|
2-8°C |
| 3 |
 |
|
 |
3998

|
 |
 |
1,2 mg/ml |
|
4°C |
| 180 |
 |
|
 |
4522

|
 |
 |
0,5 mg/ml |
|
37°C |
| 14 |
 |
|
 |
4669

|
 |
 |
8,5 mg/ml |
|
37°C |
| 21 |
 |
|
 |
4669

|
 |
 |
8,5 mg/ml |
|
37°C |
| 60 |
 |
|
 |
4669

|
 |
 |
0,5 mg/ml |
|
37°C |
| 60 |
 |
|
 |
4669

|
 |
 |
2 mg/ml |
|
21°C |
| 28 |
 |
|
 |
2051

|
 |
 |
2 mg/ml |
|
40°C |
| 6 |
 |
|
 |
2051

|
 |
 |
2 mg/ml |
|
4°C |
| 70 |
 |
|
 |
2051

|
|
|