| Type : |
Journal |
|
| Research teams : |
Bethesda - National Institutes of Health Clinical Center |
| Authors : |
Cheung YW, Vishnuvajjala BR, Morris NL, Flora KP. |
| Title : |
Stability of azacitidine in infusion fluids. |
| Reference : |
Am J Hosp Pharm ; 41: 1156-1159. 1984 |
|
| Level of Evidence : |
|
| Physical stability : |
|
| Chemical stability : |
|
| Other methods : |
|
| Comments : |
| Repeatability / reproducibility / standard range: results not provided or results outside specified values | | No comments for the degradation products | | No visual inspection | | Unencrypted results (eg graphic) |
|
List of drugs
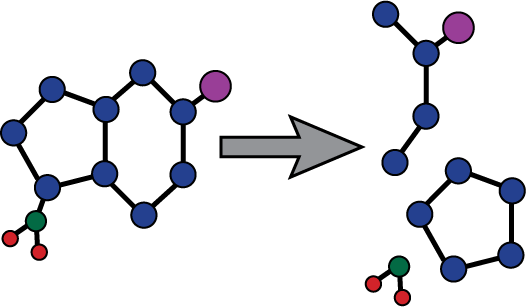
 Azacitidine Azacitidine
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
0.2 mg/ml |
25°C |
 |
| 1.9 |
 |
|
|
 |
 |
 |
2 mg/ml |
25°C |
 |
| 2.4 |
 |
|
|
 |
 |
 |
0.2 mg/ml |
25°C |
 |
| 1.9 |
 |
|
|
 |
 |
 |
2 mg/ml |
25°C |
 |
| 2.8 |
 |
|
|

|
|
| |
+
|
 |
|
|