|
 |
Tradename |
|
|
Trade names are indicative and excipients composition can be different depending on the country and manufacturers
|
|
Aggrastat |
Australia, Austria, Belgium, Canada, Denmark, Finland, Germany, Great Britain, Hungary, Iran, Ireland, Italy, Luxembourg, Malaysia, Netherlands, New Zealand, Norway, Poland, Portugal, Saudi Arabia, Sweden, Switzerland, Turkey, United Arab Emirates, United States of America |
|
Agrastat |
Argentina, Brazil, Chile, Colombia, Ecuador, Egypt, France, Mexico, Morocco, Peru, Spain, Venezuela |
|
Thrombostat |
Turkey |
|
Tirofiban |
Germany, Spain |
|
Tiroprest |
Turkey |
|
Welban |
Spain |
|
Xtraban |
India |
|
|
 |
 |
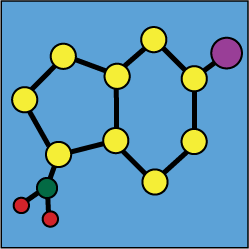
Stability of mixtures : Tirofiban |
|
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
50 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
1603

|
 |
 |
25 µg/ml |
|
25°C |
| 4 |
 |
|
 |
1420

|
 |
 |
25 µg/ml |
|
25°C |
| 4 |
 |
|
 |
1420

|
 |
 |
25 µg/ml |
|
25°C |
| 4 |
 |
|
 |
1420

|
 |
 |
25 µg/ml |
|
25°C |
| 4 |
 |
|
 |
1420

|
 |
 |
25 µg/ml |
|
25°C |
| 4 |
 |
|
 |
1420

|
 |
 |
25 µg/ml |
|
25°C |
| 4 |
 |
|
 |
1420

|
 |
 |
25 µg/ml |
|
25°C |
| 4 |
 |
|
 |
1420

|
 |
 |
25 µg/ml |
|
25°C |
| 4 |
 |
|
 |
1420

|
 |
 |
25 µg/ml |
|
25°C |
| 4 |
 |
|
 |
1420

|
 |
 |
25 µg/ml |
|
25°C |
| 4 |
 |
|
 |
1420

|
 |
 |
25 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
2004
|
 |
 |
5,5 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
2004
|
 |
 |
25 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
2004
|
 |
 |
5,5 µg/ml |
|
20°C-25°C |
| 4 |
 |
|
 |
2004
|
|
|