| tips : |
Žurnāls |
| interneta saite : |
http://www.ajhp.org/cgi/content/abstract/43/11/2803 |
|
| pētniecības grupas : |
West Point - Merck and Co |
| Autori : |
Bigley FP, Forsyth RJ, Henley MW. |
| Nosaukums : |
Compatibility of imipenem-cilastatin sodium with commonly used intravenous solutions. |
| Numurs : |
Am J Hosp Pharm ; 43: 2803-2809. 1986 |
|
| Pierādījumu līmeni : |
|
| fizikālā stabilitāte : |
|
| ķīmiskā stabilitāte : |
|
| citas metodes : |
|
| komentāri : |
| Reālos apstākļos degradēšanās produkti nav novēroti | | Rezultāti ir ar augstu mainības koeficientu vai nav nodrošināti, vai trūkst katra punkta dubultās analīzes |
|
Saraksti
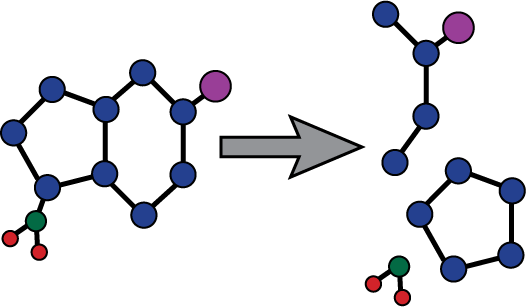
 Imipenem - cilastatin sodium Imipenem - cilastatin sodium
|
 |
 |
 |
 |
 |
 |
 |
 |
-20°C |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
2.5 mg/ml |
4°C |
 |
| 72 |
 |
|
|
 |
 |
 |
2.5 & 5 mg/ml |
25°C |
 |
| 9 |
 |
|
|
 |
 |
 |
5 mg/ml |
4°C |
 |
| 48 |
 |
|
|
 |
 |
 |
2.5 mg/ml |
4°C |
 |
| 72 |
 |
|
|
 |
 |
 |
2.5 & 5 mg/ml |
25°C |
 |
| 9 |
 |
|
|
 |
 |
 |
5 mg/ml |
4°C |
 |
| 48 |
 |
|
|
 |
 |
 |
2.5 mg/ml |
25°C |
 |
| 6 |
 |
|
|
 |
 |
 |
2.5 & 5 mg/ml |
4°C |
 |
| 24 |
 |
|
|
 |
 |
 |
5 mg/ml |
25°C |
 |
| 3 |
 |
|
|
 |
 |
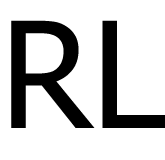 |
2.5 mg/ml |
25°C |
 |
| 6 |
 |
|
|
 |
 |
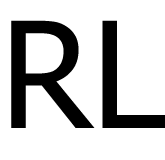 |
2.5 & 5 mg/ml |
4°C |
 |
| 24 |
 |
|
|
 |
 |
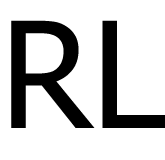 |
5 mg/ml |
25°C |
 |
| 3 |
 |
|
|
 |
 |
 |
2.5 & 5 mg/ml |
25°C |
 |
| 3 |
 |
|
|
 |
 |
 |
2.5 & 5 mg/ml |
4°C |
 |
| 24 |
 |
|
|

|
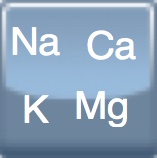
250 & 500 mg/ml |
+  Sodium bicarbonate Sodium bicarbonate
|
50 mg/ml |
| |

|
|
| |
+
|
 |
|
 Sodium bicarbonate Sodium bicarbonate
|
 |
|
|