| tüüp : |
ajaleht |
| interneti link : |
http://www.currenttherapeuticres.com/article/S0011-393X(15)00016-8/abstract |
|
| uurimisrühmad : |
San Francisco - Theravance Biopharma |
| Autor : |
Gu Z, Parra C, Wong A, Nguyen A, Cheung R, Catalano T. |
| pealkiri : |
Post-reconstitution Stability of Telavancin with Commonly Used Diluents and Intravenous Infusion Solutions. |
| publikatsioon : |
Current Ther Res ; 77: 105-110. 2015 |
|
| Tõendite tase : |
|
| füüsikaline stabiilsus : |
|
| Keemiline püsivus : |
|
| Muud meetodid : |
|
| Kommentaarid : |
| Laguproduktid tuvastatud ja kvantifitseeritud |
|
ingredientide nimekiri
 Telavancin hydrochloride Telavancin hydrochloride
|
 |
 |
 |
 |
15 mg/ml |
2-8°C |
 |
| 7 |
 |
|
|
 |
 |
 |
15 mg/ml |
20-25°C |
 |
| 12 |
 |
|
|
 |
 |
 |
15 mg/ml |
2-8°C |
 |
| 7 |
 |
|
|
 |
 |
 |
15 mg/ml |
20-25°C |
 |
| 12 |
 |
|
|
 |
 |
 |
0.6 mg/ml |
2-8°C |
 |
| 7 |
 |
|
|
 |
 |
 |
0.6 mg/ml |
20-25°C |
 |
| 12 |
 |
|
|
 |
 |
 |
8 mg/ml |
2-8°C |
 |
| 7 |
 |
|
|
 |
 |
 |
8 mg/ml |
20-25°C |
 |
| 12 |
 |
|
|
 |
 |
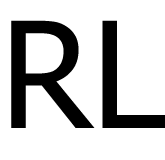 |
0.6 mg/ml |
2-8°C |
 |
| 7 |
 |
|
|
 |
 |
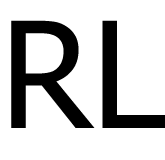 |
0.6 mg/ml |
20-25°C |
 |
| 12 |
 |
|
|
 |
 |
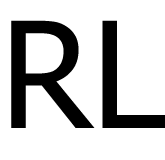 |
8 mg/ml |
2-8°C |
 |
| 7 |
 |
|
|
 |
 |
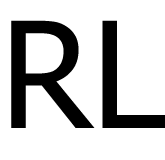 |
8 mg/ml |
20-25°C |
 |
| 12 |
 |
|
|
|
|