| Type : |
Journal |
| Web link : |
https://doi.org/10.1515/pthp-2017-0025 |
|
| Research teams : |
Mainz - University Medical Center, Johannes Gutenberg |
| Authors : |
Kim S, Heeb R, Krämer I. |
| Title : |
Physicochemical Stability of Reconstituted Decitabine (Dacogen®) Solutions and Ready-to-Administer Infusion Bags when Stored Refrigerated or Frozen. |
| Reference : |
Pharmaceutical Technology in Hospital Pharmacy ;2,4: 145-157. 2017 |
|
| Level of Evidence : |
|
| Physical stability : |
|
| Chemical stability : |
|
| Other methods : |
|
| Comments : |
| Repeatability / reproducibility / standard range: results not provided or results outside specified values |
|
List of drugs
 Decitabine Decitabine
|
 |
 |
 |
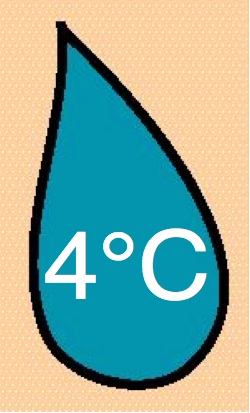 |
5 mg/ml |
2-8°C |
 |
| 12 |
 |
|
|
 |
 |
 |
0.5 mg/ml |
2-8°C |
 |
| 24 |
 |
|
|
 |
 |
 |
5 mg/ml |
-25°C |
 |
| 28 |
 |
|
|
|
|