| Type : |
Journal |
| Web link : |
https://doi.org/10.1093/ajhp/62.6.630 |
|
| Research teams : |
La Plata - Facultad de Ciencias Exactas |
| Authors : |
Volonté MG, Valora PD, Congolani A, Ferrara M. |
| Title : |
Stability of ibuprofen in injection solutions. |
| Reference : |
Am J Health-Syst Pharm ; 62: 630-633. 2005 |
|
| Level of Evidence : |
|
| Physical stability : |
|
| Chemical stability : |
|
| Other methods : |
|
| Comments : |
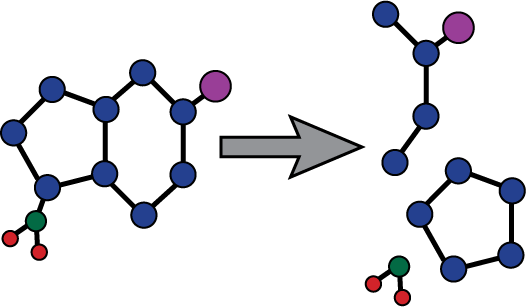
| Degradation products identified and quantified |
|
List of drugs
 Ibuprofen lysinate Ibuprofen lysinate
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
1 & 4 mg/ml |
25°C |
 |
| 15 |
 |
|
|
 |
 |
 |
27,5 mg/ml |
25°C |
 |
| 15 |
 |
|
|
 |
 |
 |
1 & 4 mg/ml |
25°C |
 |
| 15 |
 |
|
|
 |
 |
 |
1 & 4 mg/ml |
25°C |
 |
| 15 |
 |
|
|

|
|
| |
+
|
 |

|
|
| |
+
|
 |
|
|